USS San Diego ACR-6 (3/3)
Armor plating makes most warships top-heavy compared to other vessels, and as a result, they flip over when they sink, just as the San Diego has. The old hull is more or less intact, but rusting through in many places, allowing access to new parts of the interior all the time.

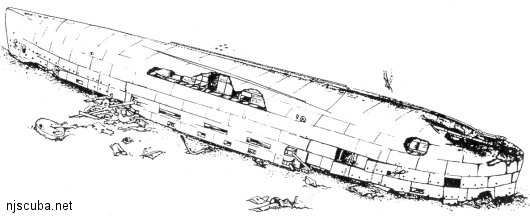
The ship is still full of live ammunition, and every so often some idiot will bring a piece of it up, resulting in a very interesting day for the local bomb squad. Explosives generally become more unstable with age, and being immersed in the water does little to change that. Souvenir shells from the San Diego could go off from a tap, or even just from drying out!
Since the Navy claims ownership of the San Diego, and because of its status as a war grave, it is strictly illegal to "salvage" any artifacts from the wreck anyway. In addition, the San Diego has recently been declared a "National Historic Site."

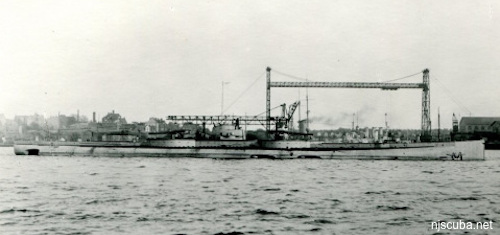

anti-aircraft light cruiser 1941-1946
541 ft, 6,000 tons
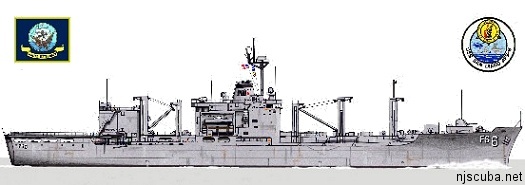
combat stores ship 1968-1997
581 ft, 17,000 tons full load

amphibious assault ship
684 ft, 24,000 tons full load


Questions or Inquiries?
Just want to say Hello? Sign the .