Water Composition (2/5)
Seawater & Salinity
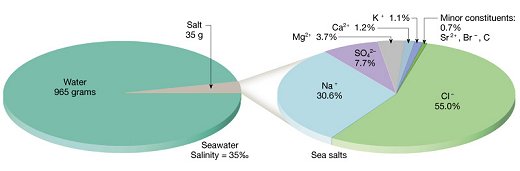
| Chloride ( Cl- ) | 55.04% wt |
| Sodium ( Na+ ) | 30.61% wt |
| Sulphate ( SO4- ) | 7.68% wt |
| Magnesium ( Mg+ ) | 3.69% wt |
| Calcium ( Ca+ ) | 1.16% wt |
| Potassium ( K+ ) | 1.10% wt |
| Total | 99.28% wt |
Seawater is a solution of salts of nearly constant composition, dissolved in variable amounts of water. There are over 70 elements dissolved in seawater but only 6 make up over 99% of all the dissolved salts; all occur as ions - electrically charged atoms or groups of atoms.
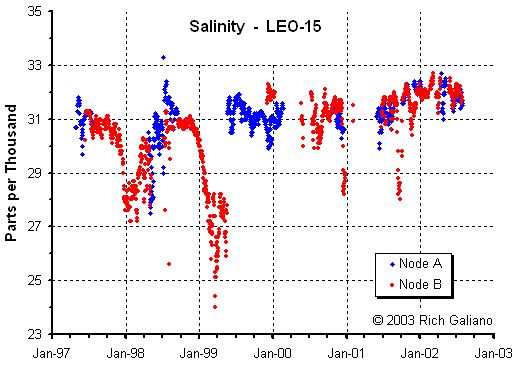
Oceanographers use salinity - the amount ( in grams ) of total dissolved salts present in 1 kilogram of water - to express the salt content of seawater. Normal seawater has a salinity of 5 parts per thousand, also expressed as 35°. This equates to a specific gravity of 1.020 to 1.024.
Seawater varies in salinity from place to place, ranging between 34° and 37° in open ocean areas, down to 0° near the discharge of large rivers. High evaporation levels cause noticeably saltier surface water in the tropics, while freshwater runoff in some enclosed northern areas like the Baltic Sea dilutes the seawater to almost fresh. Seawater from Wormly in southern England is used as the international standard for seawater composition. Locally, the salinity of coastal waters averages about 31°, or a specific gravity of 1.022. The North Atlantic is the warmest and least saline of all oceans.

As well as major elements, there are many trace elements in seawater - e.g., manganese (Mn), lead (Pb), gold (Au), iron (Fe), iodine (I). Most occur in parts per million (ppm) or parts per billion (ppb) concentrations. They are important to some biochemical reactions - both from positive and negative (toxicity) viewpoints.
Processes Controlling Seawater Composition
Salts dissolved in seawater come from three main sources:
- volcanic eruptions
- chemical reactions between seawater and hot, newly formed volcanic rocks of spreading zones ( mid-oceanic ridges )
- chemical weathering of rocks on the continents
Volcanic eruptions produce large volumes of gases that eventually reach the oceans -- most important are sulphate and chloride. Submarine eruptions at spreading ridges inject gases directly into the oceans; gases from sub-aerial volcanoes are dissolved in rainfall.
Chemical reactions between hot seawater and recently formed basaltic ocean crust lead to removal of magnesium and some sulphate from the seawater, while other elements like lithium and rubidium are added. Ocean water is circulated through the ocean crust by this exchange mechanism. The entire volume of all the ocean's water circulates every 5-10 million years. This is probably the main reason that the composition of seawater has been nearly constant over billions of years.
Many salts in seawater originate from weathering of rocks on land. As rocks are weathered to form soils, they release soluble constituents like silica and elements like sodium, calcium, potassium and magnesium. River waters also carry bicarbonate (HCO3-) - a by-product of weathering of silicate rocks or dissolution of limestone. Once they enter the oceans the dissolved salts remain, while the water continues to move through the hydrological cycle.
Most reactions occur between soil waters and silicate ( feldspars, quartz, etc. ) or carbonate ( e.g. calcite, dolomite ) minerals in rocks:
Silicate minerals + CO2 + H2O => Clay mineral + dissolved silica + ions ( sodium, calcium, bicarbonate, etc. )
Example: weathering of feldspar:
2 NaAlSi3O8 + 2 CO2 + 11 H2O = Al2Si2O5(OH)4 + 2 Na+ + 2 HCO3- + 4 H4SiO4
Limestone ( composed of the mineral calcite ) is completely dissolved by acidic waters:
CaCO3 + H2O + CO2 = Ca+ + 2 HCO3-
Because there is geological evidence to suggest that seawater has retained the same salinity for billions of years ( at least 3.4 Ga ) other processes must be removing dissolved constituents from seawater. Some are removed by chemical reactions between seawater and the sediment ( e.g. manganese nodules. ) Others ( e.g. silica, nitrate, calcium carbonate ) are removed by organisms, including skeletal material. Other constituents, like sodium and chloride are removed as salts ( evaporite deposits ) when some of the seawater evaporates. This occurs when an arm of the sea is partially or totally cut-off from the open ocean and happens to lie in an arid climatic zone. The potash deposits of Saskatchewan were formed when an arm of the sea was isolated from the open ocean about 400 million years ago. Most of the water evaporated, leaving behind a thick deposit of salts. Wind blowing sea-spray also removes salt as aerosols which may be deposited along the coastline. Also, some minerals in seafloor sediments, such as clays, adsorb ( "grab" ) metallic ions from seawater onto their surfaces. When evaporites and seafloor sediments are uplifted, associated with collision of lithospheric plates, they again become subject to weathering and the dissolved salts eventually return to the sea.
pH
pH, or "percent Hydrogen", is a measure of the acidity or alkalinity of a substance, generally a water-based mixture. pH is measured on a scale from 0 to 14. The pH scale is logarithmic - the numbers are actually exponents, but you don't need to understand how that works to understand the concept.
A pH of 7.0 is by definition neutral. Acidity ( pH < 7.0 ) is a measure of the concentration of free hydrogen ions ( H+ or simply bare protons ) in a solution. Alkalinity ( pH > 7.0 ) is a measure of the concentration of "holes" where such a hydrogen ion could fit. Since water dissociates ( breaks apart ) into equal parts of hydrogen ions and "holes" ( in this case, hydroxide ions OH-, although any negative ion will act as a base ) it is equally an acid and a base, or perfectly neutral:
H2O <=> OH- + H+
Warning:
Hydrogen hydroxide ( HOH ) is commonly used as an industrial solvent, and also in the manufacture of chemical and biological weapons. It is often present in cancerous tumors. Exposure to the gaseous form results in blisters and burns; contact with the solid form results in numbness followed by tissue damage; immersion in the liquid form has been linked to many fatalities.
Hydrogen hydroxide is water. Anything can be made to sound bad - beware of radical environmentalists !
In fact, another name for water is hydrogen hydroxide - HOH. ( Also: hydric acid, and that even more feared enviro-toxin: dihydrogen monoxide. )
Carbon dioxide is more soluble in water than most gases, owing to the polar nature of the CO2 molecule, similar in this regard to the water molecule itself. In combination with water, carbon dioxide readily forms carbonic acid, H2CO3. Carbonic acid may then dissociate to form bicarbonate ions HCO3- and hydrogen ions H+, which may further dissociate to form carbonate ions CO3- - and yet more hydrogen ions:
CO2 + H2O <=> H2CO3
H2CO3 <=> HCO3- + H+
HCO3- <=> CO3- - + H+
or
CO2 + H2O <=> H2CO3 <=> HCO3- + H+ <=> CO3- - + 2H+
Carbonate and bicarbonate are both well-known alkalines, or bases, while hydrogen ions are acidic. This series of reactions, which go equally well in either direction, forms the basis of a chemical buffering system which holds the pH ( or acidity ) of seawater to a stable and narrow range between 7.5 and 8.4. This is slightly alkaline, owing to the concentration of the strongly alkaline positive ions listed above: mainly calcium carbonates, but also similar compounds of potassium and sodium.
By comparison, pure distilled water is neutral in pH ( by definition, pH = 7.0 ) whereas most natural freshwater bodies tend toward acidity, in the range from 5.0 to 7.5. This acidity is due mainly to products of biological decomposition. In some areas, compounds leaching from fallen tree leaves, especially oak, can make the water extremely acidic, with a tea-like coloration. This is common in many areas of southern New Jersey.
For comparison, the pH of lemon juice is about 3.0; the pH of soap is about 10.


