Water Composition (3/5)
The Mid-Atlantic Bight and the Gulf Stream
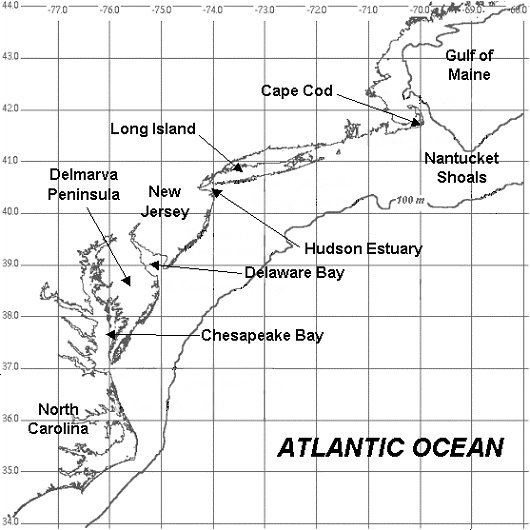
The Mid-Atlantic Bight is the region enclosed by the coastline from the North Carolina Capes in the south to Cape Cod in the north. The outer boundary is typically taken as the edge of the Gulf Stream. These latitudes are classified as cold temperate, with wide seasonal variations in temperature and solar radiation.

Of note is that the current diverges from the continental margin off the Carolinas, and passes far offshore of New Jersey. The Gulf Stream is primarily a surface current, which floats atop the colder denser deep ocean waters.
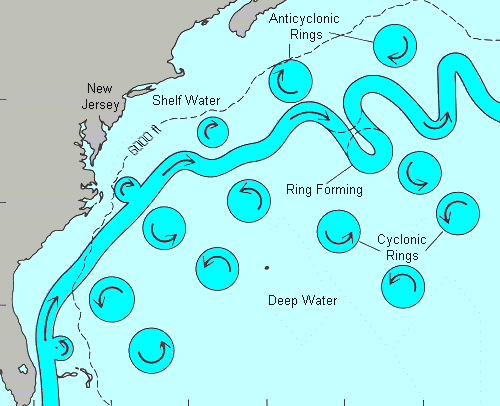
This diagram shows how loops are pinched off, forming circulating pockets of tropical water which spin away from the main flow on either side. Some of these bring warm tropical water to our shores, even though the main flow is hundreds of miles out to sea and heading for England. ( Cyclonic simply means counterclockwise, the normal direction of rotation of atmospheric storms in the northern hemisphere. )
Temperature
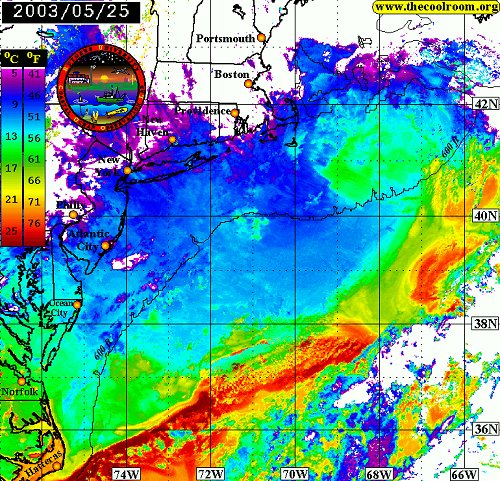

This image shows the warm Gulf Stream waters ( red ) meandering offshore, leaving the Mid-Atlantic Bight area filled with the cold water of the northern Labrador Current. Note the eddy of warm water ( yellow ) swirling away from the main flow at middle right. The water temperatures shown here are unusually cold for this time of year.
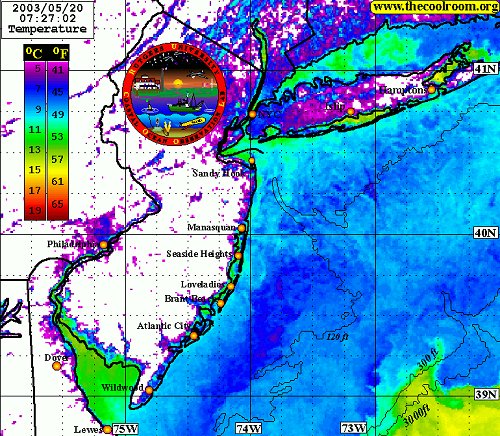
Note the bottom contours and the finger of warm water at the lower right. These warm surface waters float above colder layers below. Water composition is also influenced by freshwater river flows and runoff from the land, as is evidenced by the temperature gradients along the shore and in the shallow bays.
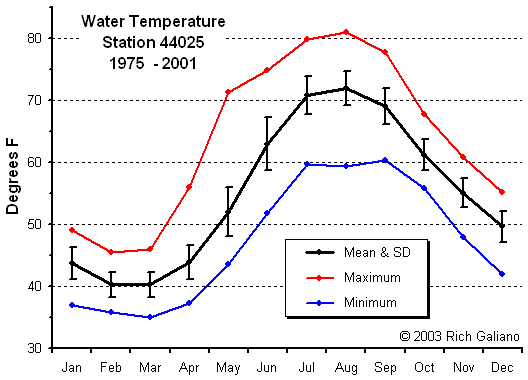

Note how bottom temperature trends along with offshore surface temperature ( above ) peaking somewhat later ( September vs. August. ) the LEO-15 stations are inshore in relatively shallow water, and these temperatures should not be considered indicative of the entire area. In particular, deep waters offshore are considerably colder in the summer months.
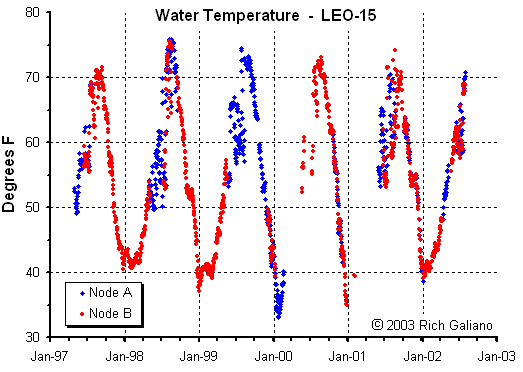

Thermoclines & Stratification
In temperate-zone seas as off the New Jersey coast, the amount of sunlight varies seasonally. As a result, the amount of solar energy entering the water varies, which in turn alters the temperature in the upper water layers. The thermal structure of the water column thus changes seasonally.

In the summer months, the sun is high, days are long, and the upper layers heat up and become less dense than the underlying layers. In other words, the water column becomes thermally stratified and no mixing occurs. In the fall the amount of solar energy entering the water column decreases, days become shorter, upper layers cool, and thermal stratification decreases. Finally, a point is reached where the temperature of the surface layers has been reduced to such an extent that the density of the layer is little different from that of the underlying mass. At this point, mixing can occur whenever sufficient wind is available.
In winter, usually the storm season in the temperate zone, the sun is lowest on the horizon, solar energy input to the water is at a minimum, thermal stratification is at a minimum or absent, and mixing occurs. With the onset of spring, the days become longer, the solar energy increases, the upper layers begin to rise in temperature, and the system moves toward re-establishment of thermal stratification.
This same sequence of events also occurs in many freshwater bodies of water.
Oxygen & Other Dissolved Gases
Seawater also contains small amounts of dissolved gases ( nitrogen, oxygen, carbon dioxide, hydrogen, and trace gases. ) Water at a given temperature and salinity is saturated with gas when the amount of gas entering the water equals the amount leaving during the same time. Surface seawater is normally saturated with atmospheric gases such as oxygen and nitrogen. The amount of gas that can dissolve in seawater is determined predominantly by the water's temperature and salinity. Increasing the temperature or salinity reduces the amount of gas that can be dissolved.
35°F seawater at the surface typically contains about 0.54% dissolved oxygen by volume at 68°F, and about 0.80% at 32°F ( compared to 21% for air ! ) Most fish become stressed when dissolved oxygen levels fall to 0.2-0.4%, and large-scale die-offs of aquatic fauna may occur at levels below 0.2%.
| Dissolved Oxygen | |||||
| Temperature | Freshwater | Saltwater 35° | |||
| deg C | deg F | saturated (100%) |
minimum healthy ( 6 mg/l ) |
saturated (100%) |
minimum healthy ( 5.5 mg/l ) |
| 0 | 32 | 14.6 mg/l | 41% | 11.7 mg/l | 47% |
| 5 | 41 | 12.8 mg/l | 47% | 10.4 mg/l | 52% |
| 10 | 50 | 11.3 mg/l | 53% | 9.3 mg/l | 58% |
| 15 | 59 | 10.1 mg/l | 59% | 8.5 mg/l | 65% |
| 20 | 68 | 9.1 mg/l | 66% | 7.8 mg/l | 71% |
| 25 | 77 | 8.2 mg/l | 73% | 7.1 mg/l | 77% |
| 30 | 86 | 7.5 mg/l | 80% | 6.5 mg/l | 85% |
A rule of thumb is that for any cold-blooded animal, the metabolic rate roughly doubles for every 10°C ( 18°F. ) Thus, a cold-blooded organism will require twice as much oxygen at 77°F as it would at 59°F, while at the same time, the available oxygen has dropped by approximately 20% ( likely more. ) Many aquatic creatures ( fishes especially ) are capable of greatly varying their respiration rate to adjust to a range of temperatures and oxygen levels.
The amount of dissolved oxygen in the water is one of the principal indicators of the health of an aquatic ecosystem. If dissolved oxygen levels drop too low, known as a Low Oxygen Event, animals which can move ( e.g., fish and lobsters ) will leave, and animals which can't ( e.g., clams and oysters ) will become stressed, and eventually die if the levels do not increase, an event known as a fishkill. There are some environments which have naturally occurring cycles of low oxygen, and some animals have evolved to tolerate the low oxygen. In some areas where the nearby coastline is heavily populated, the severity and frequency of low oxygen conditions can be directly attributed to human activities.
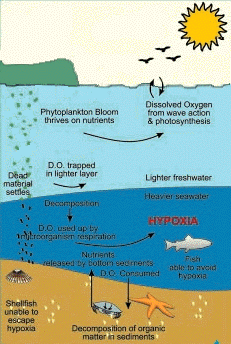
The naturally occurring processes which affect the oxygen cycle are:
- The sun and nutrients stimulate photosynthesis in marine plankton and a bloom occurs.
- As the plankton die, they rain down to the bottom and begin to decompose. The microorganisms which decompose the dead organic matter start to consume the available oxygen.
- Mixing of new dissolved oxygen from the upper waters into the lower water column is usually inhibited because the sun has also warmed the upper layer, creating a thermocline which inhibits mixing. The microorganisms continue to use up the oxygen.
- As the dissolved oxygen concentration drops below certain levels, fish and lobsters start to move out. Shellfish are unable to escape the low oxygen levels, they will die unless the oxygen levels go up again due to a major mixing event ( storms ) or the bloom ends and the number of microorganisms using up the oxygen decreases.
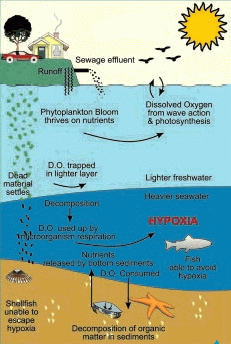
Low oxygen events like this are usually short-lived and the marine community recovers quickly. However, human processes can aggravate matters:
- The runoff from land now carries extra nutrients from lawn fertilizers and domestic animals, and effluent pipes from treatment plants add sewage and additional detrital material.
- The plankton bloom can grow faster and last much longer because of the extra nutrients.
- More dead phytoplankton and detritus is providing more material for the microorganisms on the bottom to decompose, intensifying and prolonging the dissolved oxygen depletion.
Once water sinks below the ocean surface, dissolved gases can no longer exchange with the atmosphere. The amount of gas in a given volume of water may remain unchanged, except by movement of gas molecules through the water - diffusion ( slow process ) or by the water mixing with other water masses containing different amounts of dissolved gas. In general, nitrogen and rare inert gases ( argon, helium, etc. ) behave this way - their concentrations are conservative and only affected by physical processes. In contrast, some dissolved gases are non-conservative and actively participate in chemical and biological processes that change their concentrations. Examples are oxygen and carbon dioxide - released and used at various rates in the oceans, especially by organisms.
Nitrogen, essential for plant growth, is even less soluble in water - only 0.00005% in the richest waters - 1/10,000 of the level typically found in terrestrial soil.

