Nitrox and Air

Human lungs are designed to extract the oxygen we need from air - a mixture of roughly 21% oxygen and 79% nitrogen, at a pressure of one atmosphere ( about 14.7 psia.) As you dive deeper and longer while breathing air, the increased pressure causes ever-greater amounts of both gases to dissolve in your blood and tissues. One would expect that eventually, such elevated concentrations would become troublesome, and indeed that is the case. As it turns out, nitrogen, with its greater concentration in the air, is the first gas to become a problem during a dive to recreational depths ( <130 ft. )
This problem is that of "off-gassing", or decreasing the concentration of dissolved nitrogen in the body at a rate that does not cause bubbles of the gas to form in the tissues and blood, the condition commonly known as the bends. One way to delay the onset of this problem is to decrease the concentration of nitrogen in the breathing gas, and the easiest way to do this is simply by increasing the concentration of oxygen. The resulting mixture is typically known as Enriched Air Nitrox and has become a staple in the diving community.
Nitrox mixes are named for their percentage of oxygen. Therefore, a mixture with 40% oxygen and 60% nitrogen is referred to as EANx40, while ordinary air could be referred to as EANx21. Over the years, two particular mixes have become standards for diving: EANx32 and EANx36, although many other "custom blends" are possible. The different Nitrox mixes trade-off allowable no-decompression bottom time for maximum allowable depth.
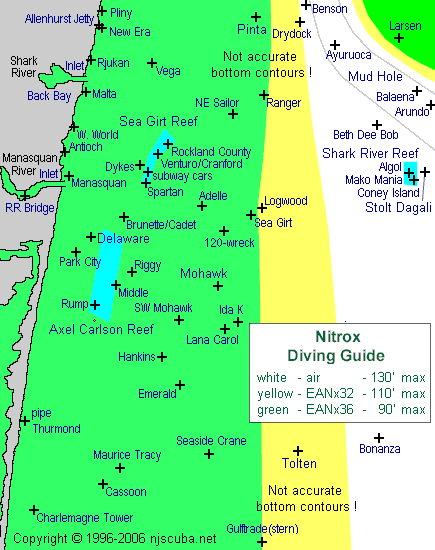
This chart shows the availability of a number of popular central New Jersey wrecks and dive sites to a diver using one of the two standard Nitrox mixes, as well as air. The contours on the chart are not accurate depths, but merely indicate which sites are within the recommended depth limits for each mixture. It is important to note is that the yellow area ( EANx32 ) also encompasses the green area, but not vice-versa. Of course, the white area ( air ) encompasses all, although there are a few places in the Mud Hole where even air cannot go.
Of course, it is assumed here that the diver will want to go to the bottom, and not just linger on the upper reaches of a wreck. After all, the sand is where most of the interesting stuff is. Otherwise, the tops of the bigger offshore wrecks like the Algol and Stolt Dagali are also available to experienced Nitrox divers who can stay above the maximum depth imposed by whatever mixture they are using.
However, diving Nitrox like this, without a "hard bottom" to limit your depth, is courting disaster. This is because the depth limits of enriched oxygen mixtures are generally based upon oxygen toxicity effects, while the depth limits for air are based on the probability of nitrogen narcosis. Nitrogen narcosis is a well-known effect that deep divers experience while breathing air at depths beyond 140-160 ft. Its onset is gradual and the effects, comparable to drunkenness, are usually manageable by experienced divers. Oxygen toxicity, on the other hand, strikes swiftly and without warning, and is usually fatal underwater - convulsions, leading to loss of consciousness and drowning, although it takes more than one or two breaths to bring it on.
Therefore, the consequences of exceeding your depth limit on Nitrox are much more serious than the consequences of exceeding your depth limit on air:
Go too deep on air, and you gradually get "narked"; go too deep on Nitrox, and you die.
( ANDI actually calls Nitrox "SafeAir". It is quite the opposite if used improperly! )
So why would you want to use Enriched Air Nitrox? Extended bottom time, that's why! Compare the PADI RDP no-decompression limits:
No Decompression Limits (minutes)
| depth | air | EANx28 | EANx32 | EANx36 |
| 50 ft | 80 | 140 | 155 | 220 |
| 60 ft | 55 | 75 | 90 | 115 |
| 70 ft | 40 | 55 | 60 | 75 |
| 80 ft | 30 | 40 | 45 | 55 |
| 90 ft | 25 | 30 | 35 | 40 |
| 100 ft | 20 | 25 | 30 | 35 |
| 110 ft | 16 | 20 | 25 | n/a |
| 120 ft | 13 | 16 | n/a | n/a |
| 130 ft | 10 | 13 | n/a | n/a |
| 140 ft | 8 | n/a | n/a | n/a |
Within its depth limits, EAN36 will almost double your bottom time, compared to air. On the chart above, you can see that even with the depth limitations of EAN36, almost all of the inshore wrecks are safely within reach, and with EAN32, only the deepest ones are out of reach. All this, for a premium of perhaps 2-3 times the cost of a regular air fill.
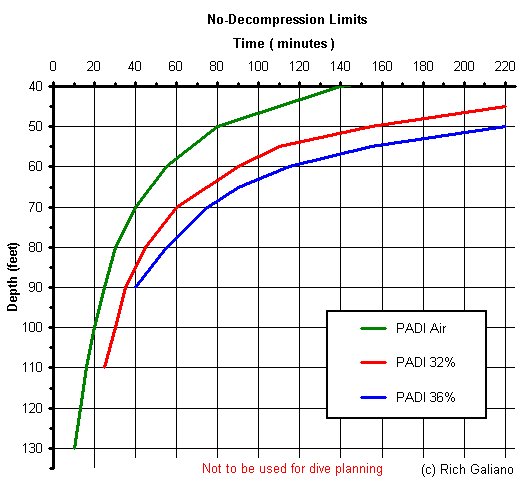
However, there are other factors which affect the Nitrox "equation". For example, how often do you actually want to stay down there that long, around here? The cold and currents might get to you long before you reach your no-decompression limit. This is especially true for a wetsuit diver, who will most likely chill and end the dive long before getting his money's worth from a bottle of Nitrox. Also, to realize the full extended bottom times that are possible, you will probably need bigger tanks - your aluminum 80 will not last any longer filled with Nitrox than it will with air.
While the extra cost of a Nitrox fill is really minor when factored into the overall cost of a day of diving ( and don't forget the value of your own time ! ) there are other equipment costs to consider when using Nitrox. For one, all your breathing equipment must be "oxygen-clean", and marked as such. This is not difficult or too expensive to accomplish, but in order to maintain this condition, you must always fill your tanks only at Nitrox fill stations, even when filling them with plain air, in which case, the shop will probably charge you the Nitrox rate anyway. Air from Nitrox fill stations is filtered more closely to avoid oils and trace contaminants that might react with the higher oxygen levels, causing your equipment to literally burn up inside. ( Well, in theory anyway. )
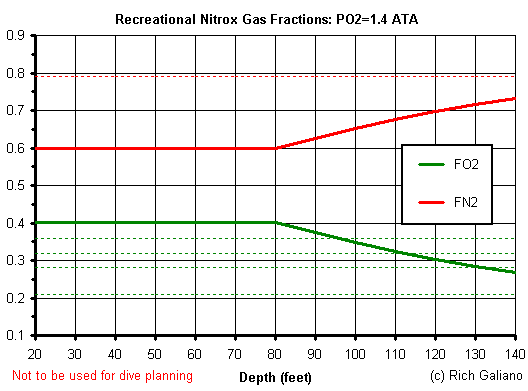
Also, to get the most benefit from using Nitrox, you should get a Nitrox-capable computer, and learn how to use it - they are more complex and expensive than air computers. A diver using air and an air computer can often safely exceed the bottom time of a diver using Nitrox and tables. Using your old air computer with Nitrox may lend a degree of safety, but it is mainly just a waste of expensive gas, and under certain conditions ( diving near the depth limits of your mix ) could expose you to oxygen poisoning.
Finally, it is vitally important that you always verify your Nitrox mix personally before using it, so that you do not accidentally exceed the depth limit of a "bad mix". While this is always done at the dive shop using oxygen test equipment that is maintained and calibrated by professionals, it is not a bad idea to have and use your own oxygen test kit as well, yet another added cost. Apart from getting bad Nitrox ( which, in truth is seldom a problem ), it is also possible to get plain old "bad air" - excessive levels of carbon monoxide can be just as fatal as oxygen, while excessive levels of contaminants like compressor oil can make you sick. Whatever you are breathing, it is important to find a good fill station that you can trust.
Many divers, especially older individuals, report feeling better during and after a dive when breathing Nitrox rather than air. Nitrox is also often credited with preventing post-dive headaches, leading to its nickname: "the feel-good gas" ( also: "geezer-gas" ). While this could be credited to the increased oxygen levels, no study has ever scientifically confirmed any of these effects. Oddly, the toxic effects of excessive oxygen are not fatal in themselves, and a diver with even acute oxygen poisoning would be expected to recover completely if rescued before drowning. At atmospheric pressure, even 100% oxygen ( EANx100 ? ) is not harmful and is in fact a standard treatment for diving accidents.
You might think that breathing all that extra oxygen would lead to elevated levels of O2 in the tissues, which could, in turn, result in "oxygen bends", but this is not the case. The bends, in its most conventional form, is a consequence of gas bubbles forming in the tissues and blood. However, since oxygen is constantly metabolized in the body, unlike nitrogen, the extremely elevated tissue levels that would be required for this do not occur. Your body simply burns off the excess oxygen before it becomes a problem.
An advanced use for Nitrox is as a decompression gas, used to speed decompression after a deep dive on air or Trimix. For this purpose, a small dedicated Nitrox "swing" bottle is carried by the diver, to assure that the decompression mix will be available even in the event of a dive gone awry. There is a danger to this, though, since several divers are known to have died from oxygen toxicity after breathing off such a bottle at the wrong depth. Another problem with this method is that it can easily push your dive computer into violation during your decompression period, even though you did a safe decompression by the tables.
Oxygen is not the only gas that can become toxic at depth. The 79% nitrogen content of air can also have a toxic effect, but this is never encountered because the narcosis effect would almost certainly prevent an air-diver from reaching the required depth alive. At those depths, generally beyond 170 ft, technical divers use Heliox / Trimix breathing gases instead of air. In fact, the well-known 130 ft recreational diving depth limit for air is actually based on the deep-diving performance of primitive early regulators and has nothing to do with the physiological effects of air at this depth.
The numbers presented here are taken directly from the PADI Nitrox Recreational Dive Planners safe recommendations. Many people today consider these numbers to be excessively conservative, especially with regard to the maximum allowable depths. In particular, it has been pointed out to me that many people consider the maximum safe depth for EANx32 to be 130 ft, rather than 110 ft. This would allow its use at all points in the chart above, except in the Mud Hole.
One problem with Nitrox is that if you plan your dive correctly and match your Nitrox mix to the depth of your intended destination, and then it changes, you are screwed. If the actual destination is shallower than the intended destination, you will not get the full benefit of your expensive gas, although you can still dive. If the actual destination is deeper than intended, you can't even dive. Given the vagaries of Northeast weather, this can be a problem as often as not! ( Note: Nitrox is ideal for whiners - "... but I don't have the right mix ..." )
36% Nitrox
It is inconsiderate and inappropriate to bring 36% Nitrox ( "whiner mix" ) on a New Jersey dive boat. 36% is limited to a safe depth of only 95 feet, which severely limits where the boat can go, and northeast weather is far too unpredictable to be hamstrung like this. By rights, if the group wants to go offshore, then the 36-percenter should be told that he is just not diving, but it never works out that way. Instead, every other person on the boat gets detoured to a destination where that one person can dive too. People with more sensible mixes, like 32%, are even more screwed, since they are now wasting their expensive fills inshore when they wanted to go offshore. 36-percenters are often left at the dock if they are caught in time. Do not bring 36% Nitrox on a dive boat unless you are the owner of the boat. Save it for the Caribbean.
My Opinion
If you are an inexperienced diver, then the extended bottom times afforded by Nitrox will only let you get into that much more trouble; ie - lost. Several times I have seen it happen that newbie divers wander off with a false sense of security due at least in part to their big tanks of Nitrox and the "training" that went with it, only to surface far from the boat - late, lost, scared, and out of gas, after a dangerous free ascent that probably put them closer to bent than they will ever be. It is my opinion that selling Nitrox training to inexperienced divers is doing them a grave and potentially dangerous disservice.
The Recreational Nitrox course itself is concerned primarily with gas theory, handling, measurements, and calculations, culminating in two "training dives" who's only purpose is to breathe out two tanks of Nitrox - you may as well do them in your bathtub. None of this will make you any better a diver in the water. In fact, if you are a beginner with inadequate skills and experience, the Nitrox course will only make you more dangerous - to yourself.

Learning to do a "hang" at the end of every dive is far more important than anything you will learn in a Nitrox class. It's easy, and it doesn't cost anything.
I am not aware of any study or statistics indicating that Nitrox use significantly reduces the likelihood of DCI. The fact is, for safety, Nitrox doesn't do anything for you that simply hanging a few extra minutes at the end of a dive won't do better. Even an "empty" ( 500 psi ) tank will last for 15-20 minutes at safety-stop depths, more than enough to do this.
While deep-diving mixes like Trimix and Heliox take you places you could not go on air, Nitrox takes you nowhere you cannot go on air. In fact, there are a lot of places you can't go on Nitrox that are perfectly accessible on air. There is no depth or diving regime where Nitrox is necessary. With proper training, experience, and discipline, very high percentages of Nitrox ( 80% ) are useful decompression gases for deep diving. However, this is the opposite of the way Nitrox is marketed to the diving masses, as a low percentage no-decompression no-brainer bottom gas!
Some people dive Nitrox as air, reckoning that this gives them a built-in safety margin. This doesn't strike me as a very good idea. It renders your dive computer totally inaccurate and excessively conservative - not a good thing while simultaneously courting possible oxygen toxicity, which, as I said above, is much more dangerous than any Nitrogen build-up. But how many people honestly have the discipline to do this anyway? In the real world, odds are, you will dive whatever Nitrox mix you have right to its limit, at which point you will be no better off than if you were using air, just a few minutes further down the road, and perhaps that much more lost. I have also seen the attitude that using Nitrox relieves you of the need to do a safety stop at the end of the dive. I find this really frightening, especially when I see it among experienced divers. Working on dive boats for many years, I have seen all of this with my own eyes.
You could argue the pros and cons of Nitrox forever. What you cannot argue is that Nitrox is extremely lucrative for dive centers, who can not only sell you the training, but then all the new equipment and maintenance to go with it, and finally the gas itself, forever, and considerably more expensive than air. As far as the diving industry is concerned, Nitrox is the greatest thing since sliced bread ( although it took PADI twenty years of steadfast refusal to finally come to this conclusion ! )
No Decompression Limits & Gas
Consumption Volume (minutes, cubic feet)
| depth | air | v | EAN x28 | v | EAN x32 | v | EAN x36 | v |
| 70 ft | 40 | 87 | 55 | 120 | 60 | 131 | 75 | 164 |
| 80 ft | 30 | 72 | 40 | 96 | 45 | 108 | 55 | 132 |
| 90 ft | 25 | 65 | 30 | 78 | 35 | 91 | 40 | 104 |
| 100 ft | 20 | 56 | 25 | 71 | 30 | 85 | 35 | 99 |
| 110 ft | 16 | 49 | 20 | 61 | 25 | 76 | ||
| 120 ft | 13 | 42 | 16 | 52 | ||||
| 130 ft | 10 | 35 | 13 | 45 | ||||
| 140 ft | 8 | 29 |
Here is the Nitrox bottom time table repeated from above, with total air consumption added, and unrealistically shallow depths deleted. This time, let's look at things from a more skeptical point of view:
36% does indeed magically almost double your bottom time at local depths, but it also limits you to 100 feet, which, as stated above, is generally unacceptable. Also, to reap the full benefit will require at least a 100 cubic foot tank, if not doubles. So users of aluminum 80s need not apply.
Do you really want to do a 75-minute dive in cold water anyway? Are you capable of it? What about your buddy? The boat captain is likely to have some choice words for you when you return from such a stunt - you will probably be invited to never come back.
32% is somewhat better, allowing most commonly encountered depths, with approximately 50% more bottom time. At greater depths, your aluminum 80 might even be usable, but at shallower depths, you will need much more capacity if you are going to get your money's worth from your expensive gas.
28% is a popular mix that is usable at almost all depths but yields only 3-5 minutes extra bottom time at the depths for which it is commonly used. How much is 3-5 minutes worth? If you want to dive deep for real, you better learn to do decompression. Again, at shallow depths, 28% greatly extends your bottom time, but only if you have big tanks.
Oh, and while you are paying triple for your fills, don't forget to add in the several hundred dollars for an oxygen analyzer, and several hundred more annually for all the O2 cleaning of your tanks and regs. Translated instead into extra dive trips, that's quite a bit of bottom time. Even on vacation in the tropics, you are likely to be using rental aluminum 80s which cannot hold enough of the precious juice to make a difference. The entire Nitrox equation makes no sense to me - the benefits are too small, if they exist at all - unless you just like to waste money.
I'm not saying that you should never become Nitrox certified. The Nitrox course can help you get a better handle on decompression theory, and having a better understanding of what is actually going on will make you a safer diver, whether or not you actually use the silly stuff.
For a seasoned diver, Nitrox is a logical next step, but not for a beginner. Unfortunately, this is not how it is marketed. Don't get talked into doing Nitrox too soon in your diving career. You are much better off using your time, effort, and money building up your experience and confidence by diving for real, and acquiring equipment that will actually make you safer and more comfortable in the water. Take a drysuit course instead.
Learning basic decompression procedures is like taking a defensive driving course - you may never plan to need it, but it might just save you someday. Teaching Nitrox to an inexperienced diver is like giving a fast car to a teenager.
Spending more money does not automatically equal SAFETY.
This is not meant to be a course in decompression theory or Enriched Air Nitrox use - I have deliberately left out all the technical details, and glossed over much of the rest. If you would like to know more, or even become Nitrox-certified, enroll in an approved Enriched Air Nitrox class at your local dive center.
I seem to be the only person in the whole world to poke holes in the Nitrox myth. I'm kinda proud of that.

