Polymer Materials (3/3)
Modern Plastics
New uses for plastics are continually being discovered. Following World War II, optical lenses, artificial eyes, and dentures of acrylic plastics, splints that X-rays may pierce, nylon fibers, machine gears, fabric coatings, wall surfacing, and plastic lamination were developed. More recently a hydrophilic, or water-attracting, plastic suitable for use in non-irritating contact lenses has been developed. Plastics reinforced with fiberglass are used for boats, automobile bodies, furniture, and building panels.


Nylon is a synthetic thermoplastic material characterized by strength, elasticity, resistance to abrasion and chemicals, low moisture absorbency, and capacity to be permanently set by heat. After 10 years of research, E. I. du Pont de Nemours & Company introduced nylon in 1938 as monofilaments for bristles and in 1940 as multifilament yarn for hosiery. Nylon is now manufactured also in the form of sheets, coatings, and molded plastics and used in a variety of products, including fabrics, surgical sutures, thread, insulating wire coverings, mosquito netting and screening, gears and bearings, rope, and tire cords. There are a variety of nylons, all being polyamides frequently made from diamines and dicarboxylic acids. The most generally useful of these is nylon-66, made from hexamethylene amine and adipic acid. Velcro is typically made of nylon.
Delrin is a polyacetal resin that was developed in 1959. It is a tough, translucent, white material that resembles nylon and polypropylene but is somewhat harder. Originally marketed as "synthetic stone", its solvent-resistant properties coupled with good dimensional stability allow Delrin to find all kinds of uses as a substitute for nonferrous metal castings in machine parts, dry-run bearings, and gears, and in other light engineering applications.

Polyester is a synthetic fiber produced by the polymerization of the product formed when an alcohol and organic acid react. The outstanding characteristic of polyesters is their ability to resist wrinkling and to spring back into shape when creased. In addition, polyesters have good dimensional stability, wash and dry easily and quickly, and have excellent wash-and-wear or minimum-care characteristics; one of their principal uses is in apparel fabrics of this kind. Microfiber, which was introduced in 1986, is a variety of polyester that has extremely thin filaments ( half as thick as silk fibers. ) Thinsulate and Polartec are polyester microfibers. Polyesters are also used in casement curtains, throw rugs, and as cushioning or insulating material. Mylar, Dacron, and Tyvek are varieties of polyester.
Polystyrene is a widely used plastic; it is a polymer of styrene. Polystyrene is a colorless, transparent thermoplastic that softens slightly above 212°F and becomes a viscous liquid at around 365°F. It is resistant to acids, alkalies, oils, and alcohols. It is produced either as a solid or as a foamed plastic marketed under the trade name Styrofoam. Its many uses include electrical and thermal insulation, translucent window panels, storage battery cases, and toilet articles.

ABS plastic is commonly used in dive gear. A wide spectrum of ABS plastics can be produced by varying the proportions of the three 3 constituent monomers - Acrylonitrile, Butadiene, and Styrene, with properties tailored to meet specific requirements. In addition to this great versatility, ABS plastics, in general, are distinguished by great toughness and high impact strength ( even at low temperatures ), good dielectric properties, and excellent dimensional stability. To this is added extremely fine gloss appearance, very wide coloring possibilities, and ready availability. All these attributes meant that when ABS was introduced in the mid-1950s, plastics could for the first time offer real competition with many traditional materials such as metals, for making highly durable moldings with great consumer appeal. One of the earliest applications was the famous Lego brick from Denmark.

Vinyl plastics are a group of thermoplastics used in molded products, flexible tubing, material for raincoats, and laminated safety glass. Vinyl plastics are polymers and copolymers of vinyl derivatives ( i.e., derivatives of ethylene, H2C-CH2, ) e.g., vinyl chloride ( H2C=CHCl ) and vinyl acetate ( H2C=CH-OOC-CH3. ) Polyvinyl chloride is an important member of this group. Polytetrafluoroethylene, or Teflon, is also sometimes classed as a vinyl polymer. Polyolefins are a group of vinyl plastics that are polymers of various alkenes, or olefins. The most important are polyethylene and polypropylene.
Polyvinyl chloride ( PVC ) is a thermoplastic that is a polymer of vinyl chloride. Resins of polyvinyl chloride are hard, but with the addition of plasticizers a flexible, elastic plastic can be made. This plastic has found extensive use as an electrical insulator for wires and cables. It is both waterproof and fire-resistant. Cloth and paper can be coated with it to produce fabrics that may be used for upholstery materials and raincoats. Most plumbing pipe is today made out of PVC. When burned, PVC materials give off a number of hazardous and polluting chlorine compounds.

Polypropylene is a plastic noted for its light weight, being less dense than water; it is a polymer of propylene. It resists moisture, oils, and solvents. Since its melting point is 250°F, it is used in the manufacture of objects that are sterilized in the course of their use. Polypropylene is also used to make textiles, ropes that float, packaging material, and luggage. For diving, polypropylene is the material of choice for floating dive flag lines and lightweight waterproof equipment boxes.
Polyethylene is the most widely used plastic. It is a polymer of ethylene H2C=CH2, having the formula (-CH2-CH2-)n, and is produced at high pressures and temperatures in the presence of any one of several catalysts, depending on the desired properties for the finished product. Polyethylene is resistant to water, acids, alkalies, and most solvents. Its many applications include films or sheets for packaging, shower curtains, unbreakable bottles, pipes, buckets and bins, drinking glasses, and insulation for wire and cable. Polyethylene products are typically labeled LDPE or HDPE, which stands for Low-Density Polyethylene and High-Density Polyethylene.
Polyurethanes are a group of plastics that may be either thermosetting or thermoplastic. Polyurethane can be made into both flexible and rigid foams. The flexible foam is often used in furniture and automobile cushions, in mattresses, and for carpet backings. The rigid foam is used for the thermal insulation of refrigerators, trucks, and buildings. In the furniture industry, the rigid foam is molded into mirror frames, chair shells, and other parts that were formerly made from wood. Some polyurethanes are highly elastic materials that are resistant to chemical attack and to abrasion. They are used in such things as solid rubber tires and shoe heels. Lycra, a fiber used in stretch clothing, is a polyurethane. Polyurethanes are also used as decorative and protective coatings, exhibiting high gloss, hardness, and toughness.

Polycarbonates are a group of clear, thermoplastic polymers used mainly as molding compounds. Polycarbonates are prepared by the reaction of an aromatic difunctional phenol with either phosgene or an aromatic or aliphatic carbonate. The commercially important polycarbonates use 2,2-bis (4-hydroxyphenol)-propane (bisphenol A) and diphenyl carbonate. This polymer is a clear plastic with a slight yellow discoloration. It has excellent electrical properties and a high impact strength.
Polyacrylics are a group of thermoplastics that are transparent and highly decorative. The polyacrylics, or acrylic plastics, are polymers ( and copolymers ) of derivatives of acrylic acid, H2C-CH-COOH. The best-known acrylic plastic is polymethyl methacrylate, sold under the trade names Plexiglas and Lucite. It takes a high polish, is clear and colorless, and is transparent to visible and ultraviolet light. Since it is thermoplastic, it can be shaped while hot to form a number of objects, such as windshields for airplanes and transparent ornamental objects. Other esters of acrylic acid and methyl acrylic acid similarly polymerize and copolymerize to transparent thermoplastics, differing somewhat in hardness and in softening temperatures. Orlon and Rayon are acrylic polymers. Rayon, based on cellulose, is one of the oldest plastics, dating to 1892. It was the first man-made fiber, originally known as "artificial silk", and it is the basis of many textiles, as well as cellophane and Scotch Tape.

Cyanoacrylate, C5H5NO2 ( or Crazy Glue ) is an acrylic resin that cures ( forms its strongest bond ) almost instantly. In glue form, the cyanoacrylate molecules ( monomers ) are suspended in an acid stabilizer which inhibits polymerization. The mixture cures within seconds on contact with water ( specifically, hydroxyl ions. ) This is convenient since virtually any object you might wish to glue will have at least trace amounts of water on its surface ( especially fingers. )
Cyanoacrylate undergoes a process called anionic polymerization: the molecules start linking up when they come into contact with water, whipping around in chains to form a durable plastic mesh. The glue thickens and hardens until the thrashing molecular strands can no longer move, resulting in an incredibly strong, permanent waterproof bond. Super-glue fuming is sometimes used in criminal investigations to detect latent fingerprints. Another interesting application is the use of cyanoacrylate to close wounds in place of stitches.

Epoxy resins are a group of synthetic resins used to make plastics and adhesives. These materials are noted for their versatility, but their relatively high cost has limited their use. High resistance to chemicals and outstanding adhesion, durability, and toughness have made them valuable as coatings. Because of their high electrical resistance, durability at high and low temperatures, and the ease with which they can be poured or cast without forming bubbles, epoxy resin plastics are especially useful for encapsulating electrical and electronic components. Epoxy resin adhesives can be used on metals, construction materials, and most other synthetic resins. They are strong enough to be used in place of rivets and welds in certain industrial applications.
Fiberglass is a thread made from glass. It is made by forcing molten glass through a kind of sieve, thereby spinning it into threads. Fiberglass is strong, durable, and impervious to many caustics and to extreme temperatures. For those qualities, fabrics woven from the glass threads are widely used for industrial purposes. Fiberglass fabrics can also be made to resemble silks and cotton and are used for curtains and drapery. A wide variety of materials are made by combining fiberglass with plastic resins. These materials, which are rust-proof, are molded into the shape required or pressed into flat sheets. Boat hulls, automobile bodies, and roofing and ceiling compositions are some of the uses to which such material is put.
Acetate is one of the most important forms of artificial cellulose-based fibers; the ester of acetic acid. The first patents for the production of fibers from cellulose acetate appeared at the beginning of the 20th century. During World War I, the production of acetylcellulose began on an industrial scale for military applications. Acetate fibers are basically delivered in the form of a continuous textile yarn. Their principal use is in the production of widely used consumer goods, such as men's shirts, women's blouses, underwear, ties, bathing suits, jersey jackets and sweaters, suit fabrics, coats, and sports clothing.
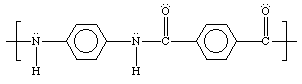
Kevlar is a high-strength synthetic fiber similar to Nylon. It was first produced by the DuPont Corporation in the early 1960s and arrived in commercial products in the 1970s. Kevlar is very strong and very light: by weight about five times as strong as steel. It is a polymeric aromatic amide, an aramid polymer containing a benzene ring, linked together through amide (nitrogen) groups. Because of the high ratio of carbon to hydrogen atoms, Kevlar requires high concentrations of oxygen before it starts to burn, leading to very low flammability. The planar aromatic rings polymerize in rigid chains, and hydrogen bonding between the hydrogen atoms of one chain, and oxygen of another leads to a strong planar sheet structure. When the material is made into fibers, the flat sheets are spun 360 degrees around the fiber axis, forming the cylindrical fiber shape. Kevlar has a high price at least partly because of the difficulties caused by the use of concentrated acid in its manufacture. An early use for Kevlar was to replace steel cords in car tires. It is now commonly used in bulletproof vests and other types of light armor and extreme sports equipment.
Silicones
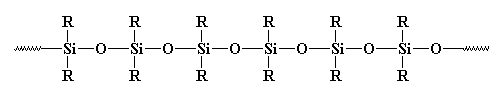

Silicone ( properly polysiloxane ) is an inorganic polymer in which atoms of silicon and oxygen alternate in a chain; various organic radicals ( the "R"s above, ) such as the methyl group, CH3, are bound to the silicon atoms. Silicones, which are unusually stable at extreme temperatures ( both high and low, ) may occur as liquids, rubbers, resins, or greases. Silicones are prepared from halides of organic silicon compounds by decomposition. Such compounds are chosen and used in mixtures that allow the desired molecular weight and degree of cross-linking to be obtained in the final polymer.
Apart from use in cast and molded products such as mouthpieces and mask skirts, silicone compounds such as RTV ( Room Temperature Vulcanize, in case you were wondering ) are also used as flexible adhesives and caulks, bonding a wide range of materials including glass, metal, and rubber. Silicone foams are used as fire barriers. Some surgical tubing is made of silicone, although most is latex. Other silicone formulations are used as lubricants and low-friction coatings. Silicones are also used to make the heat-resistant tiles on the bottom of the space shuttle, and hair conditioners that don't cause buildup. For scuba diving, silicones are commonly used to form regulator mouthpieces and mask skirts.



Silicones find uses from molded products to adhesives and caulks to lubricants to ...
Polydimethylsiloxane does something strange when mixed with boric acid, or B(OH)3. The resulting mixture is soft and pliable, and you can mold it into any shape easily with your fingers. But it is also very bouncy. What's more, push it gently and it gives way but hit it hard with a hammer and it cracks! Strangely, if you spread it over newspaper, and pull it away, it gets printed with a mirror image of the newspaper text. No industrial use was ever found for his wonder material, but tons of it has been sold as toy called Silly Putty.
Other inorganic polymers have been synthesized with backbones of pure silicone, germanium, tin, and alternating phosphorus and nitrogen atoms.
Biological Polymers

We are polymers! Or at least, we are based largely on polymer materials - proteins and nucleic acids or DNA are both organic polymers. Therefore, without polymers, there would definitely be no scuba diving.
Proteins
Protein is any of the group of highly complex organic compounds found in all living cells and comprising the most abundant class of all biological molecules. Protein comprises approximately 50% of cellular dry weight. Hundreds of protein molecules have been isolated in pure, homogeneous form; many have been crystallized. All contain carbon, hydrogen, and oxygen, and nearly all contain sulfur as well. Some proteins also incorporate phosphorous, iron, zinc, and copper. Proteins are large molecules with high molecular weights (from about 10,000 for small ones [of 50-100 amino acids] to more than 1,000,000 for certain forms); they are composed of varying amounts of the same 20 amino acids, which in the intact protein are united through covalent chemical linkages called peptide bonds. The amino acids, linked together, form linear unbranched polymeric structures called polypeptide chains; such chains may contain hundreds of amino-acid residues; these are arranged in a specific order for a given species of protein.
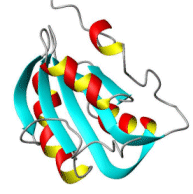
A protein molecule that consists of but a single polypeptide chain is said to be monomeric; proteins made up of more than one polypeptide chain, as many of the large ones are, are called oligomeric. Based upon chemical composition, proteins are divided into two major classes: simple proteins, which are composed of only amino acids, and conjugated proteins, which are composed of amino acids and additional organic and inorganic groupings, certain of which are called prosthetic groups. Conjugated proteins include glycoproteins, which contain carbohydrates; lipoproteins, which contain lipids; and nucleoproteins, which contain nucleic acids.
Classified by biological function, proteins include the enzymes, which are responsible for catalyzing the thousands of chemical reactions of the living cell; keratin, elastin, and collagen, which are important types of structural, or support, proteins; hemoglobin and other gas transport proteins; ovalbumin, casein, and other nutrient molecules; antibodies, which are molecules of the immune system; protein hormones, which regulate metabolism; and proteins that perform mechanical work, such as actin and myosin, the contractile muscle proteins.
Nucleic Acids
Nucleic acid is any of a group of organic substances found in the chromosomes of living cells and viruses that play a central role in the storage and replication of hereditary information and in the expression of this information through protein synthesis. In most organisms, nucleic acids occur in combination with proteins; the combined substances are called nucleoproteins. Nucleic acid molecules are complex chains of varying lengths. The two chief types of nucleic acids are DNA ( deoxyribonucleic acid ), which carries the hereditary information from generation to generation, and RNA ( ribonucleic acid ), which delivers the instructions coded in this information to the cell's protein manufacturing sites.
A substance that he called nuclein ( now known as DNA ) was isolated by 1869 by Friedrich Miescher, but it was only in the last half of the 20th century that that research revealed its significance as the material of which the gene is composed, and thus its function as the chemical bearer of hereditary characteristics. RNA was first made by laboratory synthesis in 1955. In 1965 the nucleotide sequence of tRNA was determined, and in 1967 the synthesis of biologically active DNA was achieved. The amount of RNA varies from cell to cell, but the amount of DNA is normally constant for all typical cells of a given species of plant or animal, no matter what the size or function of that cell. The amount doubles as the chromosomes replicate themselves before cell division takes place; in the ovum and sperm, the amount is half that in the body cells.
The chemical and physical properties of DNA suit it for both replication and transfer of information. Each DNA molecule is a long two-stranded chain. The strands are made up of subunits called nucleotides, each containing a sugar (deoxyribose), a phosphate group, and one of four nitrogenous bases, guanine, thymine, and cytosine, denoted A, G, T, and C, respectively. A given strand contains nucleotides bearing each of these four. The information carried by a given gene is coded in the sequence in which the nucleotides bearing different bases occur along the strand. These nucleotide sequences determine the sequences of amino acids in the polypeptide chain of the protein specified by that gene.
Between the genes, or coding loci, on the DNA of higher organisms, there are long portions of DNA, often referred to as "junk" DNA, that code no proteins. Sometimes junk DNA occurs within a gene; when this occurs, the coding portions are called exons and the noncoding (junk) portions are called introns. Junk DNA makes up 97% of the DNA in the human genome. Little is known of its purpose.
In 1953 the molecular biologists J. D. Watson, an American, and F. H. Crick, an Englishman, proposed that the two DNA strands were coiled in a double helix. In this model, each nucleotide subunit along one strand is bound to a nucleotide sub-unit on the other strand by hydrogen bonds between the base portions of the nucleotides. The fact that adenine bonds only with thymine (A-T) and guanine bonds only with cytosine (G-C) determines that the strands will be complementary, i.e., that for every adenine on one strand there will be a thymine on the other strand. It is the property of complementarity between strands that insures that DNA can be replicated, i.e., that identical copies can be made in order to be transmitted to the next generation.
compiled from various sources