Iron, Steel & Rust
Iron and steel artifacts are much less sought-after than brass and other metals, because they readily corrode in saltwater, and are difficult to clean and preserve after recovery. Exposure to air tremendously accelerates the corrosive process, and without proper conservation, a nice iron or steel artifact will become an ugly lump of rust within weeks of removal from the water.
Iron
- symbol Fe [ Latin ferrum ]
- atomic number 26
- atomic weight 55.847
- melting point about 1,535°C
- boiling point about 2,750°C
- specific gravity 7.87 at 20°C
- valence +2, +3, +4, or +6
Iron is biologically significant. Because iron is a component of hemoglobin, a red oxygen-carrying pigment of the red blood cells of vertebrates, iron compounds are important in nutrition; one cause of anemia is iron deficiency.
Properties
Iron is a lustrous, ductile, malleable, silver-gray metal ( group VIII of the periodic table. ) It is known to exist in four distinct crystalline forms. The most common is the alpha-form, which is stable below about 770°C, and has a body-centered cubic crystalline structure; it is often called ferrite. Iron is attracted by a magnet and is itself easily magnetized. It is a good conductor of heat and electricity. It displaces hydrogen from hydrochloric or dilute sulfuric acid, but becomes passive ( loses its normal chemical activity ) when treated with cold nitric acid.
Compounds
Iron forms such compounds as oxides, hydroxides, halides, acetates, carbonates, sulfides, nitrates, sulfates, and a number of complex ions. It is chemically active and forms two major series of chemical compounds, the bivalent iron (II), or ferrous, compounds and the trivalent iron (III), or ferric, compounds.
Ferrous sulfate heptahydrate, FeSO4-7H2O, sometimes called green vitriol, is a compound formed by the reaction of dilute sulfuric acid ( formerly called oil of vitriol ) with metallic iron; it is used in the manufacture of ink, in dyeing, and as a disinfectant. Ferric chloride hexahydrate, FeCl3-6H2O, is a yellow-brown crystalline compound used as a mordant in dyeing and as an etching compound. Ferric oxide, Fe2O3, is a reddish-brown powder used as a paint pigment and in abrasive rouges. Prussian blue, KFe2(CN)6, is a pigment containing the ferrocyanide complex ion. Iron rusts readily in moist air, forming a complex mixture of compounds that is mostly a ferrous-ferric oxide with the composition Fe3O4.

Natural Occurrence
Iron is an abundant element in the universe; it is found in many stars, including the sun. Iron is the fourth most abundant element in the earth's crust, of which it constitutes about 5% by weight, and is believed to be the major component of the earth's core. Iron is found distributed in the soil in low concentrations and is found dissolved in groundwaters and the ocean to a limited extent. It is rarely found uncombined in nature except in meteorites, but iron ores and minerals are abundant and widely distributed.
The principal ores of iron are hematite ( ferric oxide, Fe2O3 ) and limonite ( ferric oxide trihydrate, Fe2O3-3H2O. ) Other ores include siderite ( ferrous carbonate, FeCO3, ) taconite ( an iron silicate, ) and magnetite ( ferrous-ferric oxide, Fe3O4, ) which often occurs as a white sand. Iron pyrite ( iron disulfide, FeS2 ) is a crystalline gold-colored mineral known as fool's gold. Chromite is a chromium ore that contains iron. Lodestone is a form of magnetite that exhibits natural magnetic properties.
Production and Refining
Iron is produced in the United States chiefly from oxide ores. For many years rich hematite ores were produced by open-pit mining in the Mesabi Range near Lake Superior. However, these ores have been largely depleted, and iron is now produced from low-grade ores that are treated to improve their quality; this process is called beneficiation.
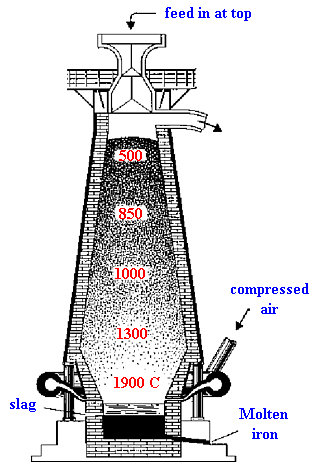
Iron ores are refined in the blast furnace (right) in a process known as smelting. The principle involved in this means of extracting metals is that of the reduction of the ores by the action of carbon monoxide, i.e., the removal of oxygen from the metal oxide in order to obtain the metal. Blast furnaces differ in construction. The one used in the production of iron consists of a chimney-like structure ( usually 80-100 ft high ) made of iron or steel and lined with firebrick. It is narrow at the top, increasing in diameter downward, but narrowing again suddenly almost at the bottom, to form the hearth or crucible. There the fine molten products are caught.
The furnace is fed from the top with a charge of definite quantities of ore, coke, and a flux, mostly limestone. Preheated compressed air is introduced at the bottom through pipes (tuyeres) entering just above the hearth. The air passes upward through the charge. The coke is oxidized to carbon dioxide, which changes to carbon monoxide at the high temperature. The carbon monoxide then reduces the ores and, taking on oxygen, reverts to carbon dioxide. This gas, together with unused carbon monoxide, nitrogen, and other constituents of the air originally introduced, is led off through a pipe from the top of the furnace and, being still at a high temperature, is employed to heat the stoves into which fresh air for the process is brought.
As the operation proceeds, the mass in the furnace becomes molten and descends into the crucible. The iron sinks to the bottom; impurities, called the slag, being lighter, float on top. The slag is drained through a pipe in the upper portion of the crucible. The iron is tapped from below and run into sand molds to harden. The product is known as pig iron or cast iron and contains about 4% carbon and small amounts of manganese, silicon, phosphorus, and sulfur. About 95% of this iron is processed further to make steel, often by the open-hearth process or the Bessemer process, but more recently in the United States and other countries by the basic oxygen process or by an electric arc furnace. The balance is cast in sand molds into blocks called pigs. It is further processed in iron foundries. Efforts to increase production rates have led to the addition of pure oxygen and steam and the sizing of ore to obtain better gas-solid contact. Flux and ore are sometimes combined into pellets. Pig iron prepared in the blast furnace is converted into steel by the Bessemer process.
Cast Iron
Cast iron is made when pig iron is re-melted in small cupola furnaces ( similar to the blast furnace in design and operation ) and poured into molds to make castings. It usually contains 2% to 6% carbon. Scrap iron or steel is often added to vary the composition. Cast iron is used extensively to make machine parts, engine cylinder blocks, stoves, pipes, steam radiators, and many other products.
Gray cast iron, or gray iron, is produced when the iron in the mold is cooled slowly. Part of the carbon separates out in plates in the form of graphite but remains physically mixed in the iron. Gray iron is brittle but soft and easily machined. White cast iron, or white iron, which is harder and more brittle, is made by cooling the molten iron rapidly. The carbon remains distributed throughout the iron as cementite ( iron carbide, Fe3C. ) Low grade cast iron is often referred to as "pot metal".
A malleable cast iron can be made by annealing white iron castings in a special furnace. Some of the carbon separates from the cementite; it is much more finely divided than in gray iron. A ductile iron may be prepared by adding magnesium to the molten pig iron; when the iron is cast the carbon forms tiny spherical nodules around the magnesium. Ductile iron is strong, shock-resistant, and easily machined.
Wrought Iron
Wrought iron is commercially purified iron. In the Aston process, pig iron is refined in a Bessemer converter and then poured into molten iron silicate slag. The resulting semisolid mass is passed between rollers that squeeze out most of the slag. The wrought iron has a fibrous structure with threads of slag running through it; it is tough, malleable, ductile, corrosion-resistant, and melts only at high temperatures. It is used to make rivets, bolts, pipes, chains, and anchors, and is also used for ornamental ironwork.
Steel
Steel is an alloy of iron, carbon, and small proportions of other elements. Iron contains impurities in the form of silicon, phosphorus, sulfur, and manganese; steelmaking involves the removal of these impurities, known as slag, and the addition of desirable alloying elements.
Production
Steel was first made by cementation, a process of heating bars of iron with charcoal in a closed furnace so that the surface of the iron acquired a high carbon content. The crucible method, originally developed to remove the slag from cementation steel, melts iron and other substances together in a fire-clay and graphite crucible. The famous blades of Damascus and of Toledo, Spain, were made by the cementation and crucible techniques. Today, the Bessemer process, the open-hearth process, and the basic oxygen process are more widely used in modern steelmaking.
The principle involved in the Bessemer process ( for Sir Henry Bessemer ) is that of oxidation of the impurities in pig iron by the oxygen of air that is blown through the molten iron; the heat of oxidation raises the temperature of the mass and keeps it molten during operation. The process is carried on in a large container called the Bessemer converter, which is made of steel and has a lining of silica and clay or of dolomite. The capacity is from 8 to 30 tons of molten iron; the usual charge is 15 or 18 tons.
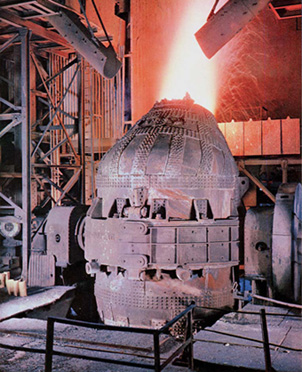
The converter is egg-shaped. At its narrow upper end, it has an opening through which the iron to be treated is introduced and the finished product is poured out. The wide end, or bottom, has a number of perforations (tuyeres) through which the air is forced upward into the converter during operation. The container is set on pivots (trunnions) so that it can be tilted at an angle to receive the charge, turned upright during the "blow, " and inclined for pouring the molten steel after the operation is complete. As the air passes upward through the molten pig iron, impurities such as silicon, manganese, and carbon unite with the oxygen in the air to form oxides; the carbon monoxide burns off with a blue flame and the other impurities form slag.
Dolomite is used as the converter lining when the phosphorus content is high; the process is then called basic Bessemer. The silica and clay lining is used in the acid Bessemer, in which phosphorus is not removed. In order to provide the elements necessary to give the steel the desired properties, another substance ( often spiegeleisen, an iron-carbon-manganese alloy ) is usually added to the molten metal after the oxidation is completed. The converter is then emptied into ladles from which the steel is poured into molds; the slag is left behind. The whole process is completed in 15 to 20 min. The Bessemer process was superseded by the open-hearth process.
The open-hearth method of steel production uses a type of furnace called a regenerative furnace; instead of a firebox at one end and a flue at the other, it has devices at each end for the intake and outflow of both fuel and air. The air is preheated by a system of current reversals that causes very high temperatures. This process, developed circa 1866 by Sir William Siemens, uses iron ore and pig iron.
In the basic oxygen process, or Linz-Donawitz process, developed in the 1950s, the design of the furnace is changed, and oxygen added to the air intake permits more rapid refining of the charge ( material in the furnace. ) the electric-arc furnace is another modern development; it provides a means of making large quantities of high-grade steel, with the advantages of positive temperature control, freedom from contamination of the product by the fuel, and simultaneous deoxidation and desulfurization actions.
Steel is shaped for commercial use in rolling mills, where successive passages of the red-hot ingot between variously shaped rollers give it the desired form. Pittsburgh, one of the world's great steel centers, built its first rolling mill in 1811; Bessemer steel rails were rolled in Chicago as early as 1865.
Types and Uses


Steel is often classified by its carbon content: a high-carbon steel is serviceable for dies and cutting tools because of its great hardness and brittleness; low- or medium-carbon steel is used for sheeting and structural forms because of its amenability to welding and tooling. Alloy steels, now most widely used, contain one or more other elements to give them specific qualities. Aluminum steel is smooth and has a high tensile strength. Chromium steel finds wide use in automobile and airplane parts on account of its hardness, strength, and elasticity, as does the chromium-vanadium variety.
Nickel steel is the most widely used of the alloys; it is nonmagnetic and has the tensile properties of high-carbon steel without the brittleness. Nickel-chromium steel possesses a shock-resistant quality that makes it suitable for armor plate. Wolfram ( tungsten ), molybdenum, and high-manganese steel are other alloys. Stainless steel, which was developed in England, has a high tensile strength and resists abrasion and corrosion because of its high chromium content.
Stainless Steel
Iron and steel, though extremely strong, are both subject to deteriorating into rust through oxidation. Stainless steels are iron-based alloys containing at least 10.5% chromium. They achieve their stainless characteristics through the formation of an invisible and adherent chromium-rich oxide film. Stainless steel is rust-resistant, even effectively rust-proof. Some types are also non-magnetic.

Unfortunately, the gain in rust-proofing is offset by a decline in the ease with which the compound can be worked and made into things. If there is much less chromium than 13%, the compound is still subject to rust. If there is much more than 13%, it is hard to work. Certain sulfides added to these materials can make the metal easier to work, however, they also subvert the rust-proof ( stainless ) quality. Stainless steels are classified into numbered grades having different properties. Some of the commonest types of stainless steels are:
Grade 304 is the standard "18/8" stainless; it is the most versatile and most widely used stainless steel, available in a wider range of products, forms, and finishes than any other. 304 SS is commonly used for chemical processing equipment, for food, dairy, and beverage industries, for heat exchangers, and for the milder chemicals. It has excellent forming and welding characteristics. The grain structure of Grade 304 enables it to be severely deep drawn without intermediate annealing, which has made this grade dominant in the manufacture of drawn stainless parts such as sinks, hollow-ware, and saucepans. For these applications, it is common to use special "304DDQ" (Deep Drawing Quality) variants. Grade 304 is also readily brake- or roll-formed into a variety of components for applications in the industrial, architectural, and transportation fields. Grade 304 also has outstanding welding characteristics. Post-weld annealing is not required when welding thin sections.
Grade 316 stainless steel contains molybdenum to control pit type attack so it is slightly more corrosion-resistant than Type 304 stainless steel. Type 316 is ideal for chemical and pulp handling equipment, for photographic equipment, food and beverage processing, and for dispensing equipment and equipment that will be exposed to saltwater. Type 316L is an extra-low carbon modification of Type 316 stainless steel used when applications are welded. Fabrication methods also influence the quality and durability of stainless steel products. The argon shield in helio-arc welding prevents oxidation and ensures a cleaner, stronger weld.
Grade 440 is capable of attaining, after heat treatment, the highest strength, hardness, and wear resistance of all the stainless alloys. Its very high carbon content is responsible for these characteristics, which make 440°C particularly suited to such applications as ball bearings and valve parts. Grades 440A and 440B are identical except for slightly lower carbon contents ( 0.60 - 0.75% and 0.75 - 0.95% respectively ); these have lower attainable hardnesses but slightly higher corrosion resistance. Although all three versions of this grade are standard grades, in practice 440°C is more available than the A or B variants. 440 stainless steels are optimized for high hardness, and other properties are to some degree compromised. Fabrication must be by methods that allow for poor weldability and usually also allow for a final harden and temper heat treatment. Corrosion resistance is lower than the common austenitic grades, and their useful operating temperature range is limited by their loss of ductility at sub-zero temperatures and loss of strength by over-tempering at elevated temperatures.
| Components | Type 304 | Type 316 | Type 316L | Type 440 |
| Chromium | 18-20% | 16-18% | 16-18% | 16-18% |
| Nickel | 8-10.5% | 10-14% | 10-14% | - |
| Carbon | 0.08% | 0.08% | 0.03% | 0.08% |
| Manganese | 2% | 2% | 2% | 1% |
| Silicon | 1% | 1% | 1% | 1% |
Rust
By far the most important form of corrosion is the rusting of iron and steel. Only Iron and steel rust; other metals corrode. Rusting is essentially a process of oxidation in which iron combines with water and oxygen to form rust, the reddish-brown crust that forms on the surface of the iron. Rust, a chemical compound, is a hydrated ferric oxide Fe2O3nH2O, where n is usually 3/2. The chemical mechanism of rusting is not fully known but is thought to involve oxidation of metallic iron to ferrous ion ( Fe++ ) and the reaction of the ferrous ion with oxygen and water to form rust. The reaction is catalyzed by water, acids, and metals below iron in the electromotive series ( e.g., copper and tin. ) Because iron is so widely used in building construction and in tools, its protection against rusting is important.
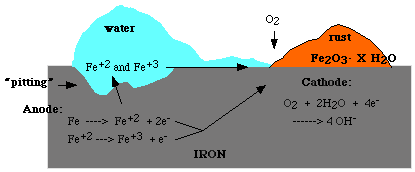
Although metals ( e.g., aluminum, chromium, and zinc ) above iron in the electromotive series corrode more readily than iron, their oxides form a tenuous coating that protects the metal from further attack. Rust is brittle and flakes off the surface of the iron, continually exposing a fresh surface. Rusting can be prevented by excluding air and water from the iron surface, e.g., by painting, oiling, or greasing, or by plating the iron with a protective coating of another metal. Metals used for plating include chromium, nickel, tin, and zinc. Zinc plating is called galvanizing.
Many alloys of iron are resistant to corrosion. Stainless steels are alloys of iron with such metals as chromium and nickel; they do not corrode because the added metals help form a hard, adherent oxide coating that resists further attack. The iron hulls of ships can be protected against rusting by attaching magnesium or zinc to the underside of the vessel. An electric current is generated, with the magnesium and iron acting as electrodes and seawater acting as the electrolyte. Because magnesium is above iron in the electromotive series, it serves as a "sacrificial anode" and is oxidized in preference to the iron. This is called cathodic protection, since the iron serves as the cathode and thus escapes oxidation. This method is also used to protect the pipes of electric generating plants where saltwater is used as a coolant.
What we normally call rust is a flaky red-brown solid which is largely hydrated iron. The primary corrosion product of iron is Fe(OH)2 ( or more likely FeO.nH2O ), but the action of oxygen and water can yield other products having different colors:
- Fe2O3nH2O ( hydrous ferrous oxide, sometimes written as Fe(OH)3 ) is the principal component of red-brown rust. It can form a mineral called hematite
- Fe3O4nH2O ( "hydrated magnetite" or ferrous ferrite, Fe2O3FeO ) is most often green but can be deep blue in the presence of organic complexants
- Fe3O4 ("magnetite") is black
 Red-brown flaky rust |
 Black magnetite |
 Blue/green unstable rust |
compiled from various sources