Copper, Brass & Bronze

Copper, brass, and bronze are all relatively immune to saltwater corrosion. Brass artifacts of all sorts are easily cleaned up into shiny souvenirs for those who value them. Bright green copper sheets and tubes add color to many wrecks, while bronze is the material of choice for the most coveted of all diver's artifacts - a ship's bell.
Copper and some of its alloys have been used by humanity since the Bronze Age. One of the first metals known to humans, free copper was probably mined in the Tigris-Euphrates valley as long ago as the 5th century BC. Cyprus, from which the metal's name originally comes, was the primary source of copper in the ancient world.
Copper
Properties
- symbol Cu [ Latin cuprum=copper ]
- atomic number 29
- atomic weight 63.546
- melting point 1,083.4°C
- boiling point 2,567°C
- specific gravity 8.96 at 20°C
- valence +1 or +2
Copper is a reddish metal with a face-centered cubic crystalline structure. It is malleable, ductile, and an extremely good conductor of both heat and electricity. It is softer than iron but harder than zinc and can be polished to a bright finish. It is found in group Ib of the periodic table, together with silver and gold.
Copper has low chemical reactivity. In moist air it slowly forms a greenish surface film ( usually a mixture of carbonate, sulfate, hydroxide, and oxide ) called patina; this coating protects the metal from further attack. Copper dissolves in hot concentrated hydrochloric or sulfuric acid but is little affected by cold solutions of these acids; it also dissolves in nitric acid. Saltwater corrodes copper, forming a chloride.

Compounds
The most important chemical compound of copper is copper sulfate pentahydrate, also called bluestone or blue vitriol. Other compounds include Paris green, Bordeaux mixture, a cyanide, a chloride, oxides, and a basic carbonate. Verdigris is basic copper acetate.
Sources and Ores
Small amounts of copper are found uncombined, particularly near Lake Superior in Michigan. Copper ores are found in various parts of the world. In the United States ( the chief producer of copper ) ores are mined in Arizona, Utah, Montana, New Mexico, Nevada, and Michigan. Copper ores are also found in Canada, South America ( in Chile and Peru, ) south-central Africa, Russia ( in the Ural mountains. ), and to a limited extent in Europe and the British Isles.
The principal ore of copper is chalcopyrite, a sulfide of copper and iron also called copper pyrite. Other important ores are chalcocite, or copper glance, a shiny lead-gray copper sulfide; bornite, a lustrous reddish-brown sulfide of copper and iron; cuprite, a red cuprous oxide ore; and malachite, a bright green carbonate ore. Azurite is a blue crystalline basic carbonate of copper found with other copper ores. Chrysocolla is a bluish-green copper silicate ore. Another important source of copper is secondary ( scrap ) copper, which is produced from discarded copper and copper alloys.
Commercial Preparation
Copper metal is prepared commercially in various ways. Copper sulfide ores, usually containing only 1% to 2% copper, are concentrated to 20% to 40% copper by the flotation process. They are then usually roasted to remove some of the sulfur and other impurities, and then smelted with iron oxide in either a blast furnace or a reverberatory furnace to produce copper matte, a molten solution of copper sulfide mixed with small amounts of iron sulfide. The matte is transferred to a converter, where it is treated by blowing air through it to remove the sulfur ( as sulfur dioxide, a gas ) and the iron ( as a slag of ferrous oxide. ) the resulting copper is 98% to 99% pure; it is called blister copper because its surface is blistered by escaping gases when it solidifies during casting.
Most copper is further purified by electrolysis. The blister copper is refined in a furnace and cast into anodes. Thin sheets of pure copper are used as cathodes. A solution of copper sulfate and sulfuric acid is used as the electrolyte. When the anode and cathode are immersed in the electrolyte and an electric current is passed, the anode is dissolved in the electrolyte, and pure copper metal is deposited on the cathode. Soluble impurities, usually nickel and arsenic, remain dissolved in the electrolyte. Insoluble impurities, often including silver, gold, and other valuable metals, settle out of the electrolyte; they may be collected and purified.
Copper oxide ores are usually treated by a different process, called leaching, in which the copper in the ore is dissolved in a leaching solution ( usually dilute sulfuric acid; ) pure copper is recovered by electrolysis. Alternatively, the solution is treated with iron to precipitate the so-called cement copper, which is impure.
Importance and Uses

Copper is present in minute amounts in the animal body and is essential to normal metabolism. It is a component of hemocyanin, the blue, oxygen-carrying blood pigment of lobsters and other large crustaceans. It is needed in the synthesis of hemoglobin, the red, oxygen-carrying pigment found in the blood of humans, although it is not a component of hemoglobin.
The chief commercial use of copper is based on its electrical conductivity ( second only to that of silver; ) about half the total annual output of copper is employed in the manufacture of electrical apparatus and wire. Copper is also used extensively as roofing, in making copper utensils, and for coins and metalwork. Copper tubing is used in plumbing, and, because of its high heat conductivity, in heat-exchanging devices such as refrigerator and air-conditioner coils. Powdered copper is sometimes used as a pigment in paints.
An important use of copper is in alloys such as brass, bronze, gunmetal, Monel metal, pot metal, and German silver. Compounds of copper are widely used as insecticides and fungicides; as pigments in paints; as mordants ( fixatives ) in dyeing; and in electroplating.
Brass

Brass is an alloy having copper ( 55-90% ) and zinc ( 10%-45% ) as its essential components. The properties of brass vary with the proportion of copper and zinc and with the addition of small amounts of other elements, such as aluminum, lead, tin, or nickel.
In general, brass can be forged or hammered into various shapes, rolled into thin sheets, drawn into wires, and machined and cast. Its ductility reaches a maximum with about 30% zinc and its tensile strength with 45% although this property varies greatly with the mechanical and heat treatment of the alloy.
Cartridge brass ( 70% copper, 30% zinc ) is used for cartridge cases, plumbing and lighting fixtures, rivets, screws, and springs. Aluminum brass ( not exceeding 3% aluminum ) has greater resistance to corrosion than ordinary brass. Brass containing tin ( not exceeding 2% ) is less liable to corrosion in seawater; it is sometimes called naval brass and is used in naval construction. Dutch metal ( 80-85% copper, 15-20% zinc ) is used as a substitute for gold leaf.
When iron is added to brass it produces hard, tough alloys. One of these is delta metal ( 55% copper, 41% zinc, 1-3% iron, and fractional percentages of tin and manganese, ) which can be forged, rolled, or cast and is used for bearings, valves, portholes, and ship propellers.

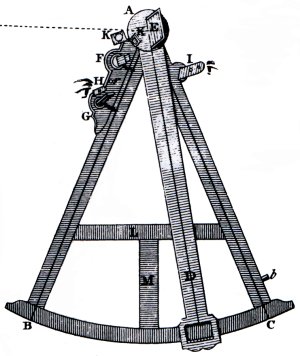
A quadrant like this would be one of a ship's officer's more prized possessions. The device was used to determine the angle of celestial bodies above the horizon and was sighted using mirrors as shown below. Two of the four mirror mounts are still evident, although the glass itself is gone.
This type of quadrant ( also referred to as an octant ) was invented in 1731. This particular one was originally found in a leather case embossed 1759. These devices were made obsolete by accurate sea-going timepieces and more-modern sextants, and went out of use in the nineteenth century.
Along with a deadeye also found on the wreck, this artifact would indicate that the SW Mohawk is most likely an old sailing ship, rather than a barge, which would have no need of navigational equipment or rigging.
Bronze
Bronze is an alloy of copper, tin, zinc, phosphorus, and sometimes small amounts of other elements. Bronzes are harder than brasses. Most are produced by melting the copper and adding the desired amounts of tin, zinc, and other substances.
The properties of the alloy depend on the proportions of its components. Aluminum bronze has high strength and resists corrosion; it is used for bearings, valve seats, and machine parts. Leaded bronze, containing from 10% to 29% lead, is cast into heavy-duty bushings and bearings. Silicon bronze is used for telegraph wires and chemical containers. Phosphor bronze is used for springs.

Bronze is used for coins, medals, steam fittings, and gunmetal and was formerly employed for cannon. Because of its particularly sonorous quality, bell metal, containing from 20% to 24% tin, is used for casting bells. Bronze has long been used in art, e.g., for castings, engravings, and forgings.

